Struck by a brilliant idea for a medical device? Maybe it’s a new surgical tool that streamlines procedures or a patient monitoring system that offers real-time insights. The possibilities are endless.
But taking that initial concept from a sketch to a real, functional product can feel like a daunting leap. That’s where medical device prototyping comes in. It’s the bridge between your innovative vision and a tangible product that helps you validate your design, gather feedback, and mitigate any product development challenges.
In this post, we’ll delve into how medical device prototyping works and some real-life examples of successful medical device prototypes.
Table of Contents
What Is Medical Device Prototyping?
Medical device prototyping is the process of creating a physical or digital three-dimensional model of your medical device concept. Medical prototyping allows you to visualize your design tangibly, test its functionality in a simulated environment, and gather feedback from potential users and stakeholders.
Here are some examples of types of prototypes used for medical devices:
- A mobile app that uses a phone’s camera to monitor chronic skin conditions, allowing patients to track progress at home
- A foam prototype of a new grip design for a surgical tool, allowing the team to assess comfort and maneuverability before investing in a more complex model
- A 3D-printed model of a custom dental implant, allowing for precise pre-surgical planning and ensuring a perfect fit before manufacturing the actual implant.
![]()
How Does Medical Device Prototyping Work?
Medical device prototyping is an iterative process that involves translating a concept into a physical model. Here’s a simplified overview of the process.
1. Creating the Concept
A team of healthcare professionals, engineers, and designers identifies a specific medical need — it could be a cumbersome diagnostic procedure or a lack of user-friendly treatment options. Through in-depth research, they explore existing solutions and brainstorm possibilities for a more efficient, user-friendly, or cost-effective approach.
For example, a group of doctors might recognize a need for better monitoring of patients in remote areas. They brainstorm and research, leading to the idea of a wearable device called Vitality Pro that can track vital signs and send data to healthcare providers in real time.
2. Design and Engineering
In the product design stage, engineers translate the team’s ideas into detailed technical drawings, considering factors like material selection, functionality, and user comfort. Human factors design prioritizes ergonomics and intuitive design to ensure the device is comfortable and easy for healthcare professionals and patients to use in real-world settings, while ensuring it performs its intended tasks effectively.
Engineers also consider safety and regulatory compliance as part of their process, including the chemical compatibility of plastics that could be exposed to alcohol or other cleaning chemicals during regular wipe-downs.
For example, based on the doctors’ concept, engineers create detailed sketches and 3D models of Vitality Pro, considering factors like size, weight, comfort, and battery life while specifying the type of sensors needed to track vital signs and the communication technology for data transmission.
3. Fabricating the Prototype
With a blueprint in hand, it’s time to fabricate the prototype. This initial model may not be perfect aesthetically, but it’s a starting point. Teams can employ techniques like 3D printing, machining, or using readily available components to build a tangible model.
The focus at this stage is on functionality — you need to test the core functionalities of the device to see if it performs as intended. This allows the team to identify any potential flaws or areas for improvement before investing significant resources in a polished final product.
For example, employing 3D printing, the prototyping engineers create a prototype casing. Electronic components like sensors and microprocessors are sourced and assembled following the engineering plans.
4. Assembly and Integration
The next stage involves assembly and integration. This is like putting together a puzzle and carefully assembling the various parts to ensure seamless operation. Here, the engineering team verifies that all components work together effectively without technical glitches. This meticulous assembly ensures the final device functions as a cohesive unit.
For example, the engineering team brings Vitality Pro to life by assembling its various components. The 3D-printed casing, designed for comfort and a snug fit, becomes the foundation. Tiny sensors for heart rate and temperature — the eyes and ears of the device — are strategically placed within the casing. The team then integrates the microprocessor, the brain of Vitality Pro, which processes the raw data collected by the sensors and prepares it for transmission.
5. Testing and Evaluation
Once assembled, healthcare professionals or potential users rigorously evaluate the prototype in simulated or controlled environments. This stage is all about gathering valuable feedback on the user interface, performance, and durability of the device, with users commenting on factors like comfort, ease of use, and effectiveness. This feedback then informs the iterative refinement stage, where the team makes changes based on the testing results.
This process can span months or even years in large medical companies and often involves a global network of test subjects.
For example, the Vitality Pro undergoes simulated scenarios where volunteers wear it while the development team evaluates its accuracy in measuring vital signs, assessing factors like user comfort, battery life, and data transmission reliability during simulated use.
6. Iterative Refinement
Iterative refinement is a crucial stage in the medical device design process where the prototype undergoes continuous improvement based on real-world testing and feedback. Think of it like sculpting a piece of clay — with each iteration, you remove imperfections and refine the shape until you achieve the desired form.
For example, based on test results, the engineers might adjust the design of the Vitality Pro to improve comfort by making it lighter or redesign the casing for a better fit.
7. Verification and Validation
Next, there’s a final round of rigorous testing to confirm it meets the specified design requirements and functions as intended.
The verification process ensures that the prototype is made according to the established specifications, while validation ensures it performs correctly in real-world conditions. This comprehensive testing process helps identify and rectify any discrepancies or issues before moving forward with production.
For instance, once the company has the final Vitality Pro product ready, it is time for one last test. The company may hire people or gather volunteers to wear the device to ensure it is functional and ready for the final stages.
8. Regulatory Compliance
Before reaching the target market (patients, doctors, or medical institutions), the device needs a green light from regulatory bodies. These agencies — such as the FDA — exist to safeguard patient safety and ensure medical devices are effective.
The prototype is assessed against established laws, regulations, and policies. This might involve documentation review and rigorous material testing to ensure biocompatibility and adherence to specific manufacturing standards.
For example, the Vitality Pro might need 510(k) clearances from the FDA before it’s ready to sell.
9. Production Scaling
With the prototype perfected and regulatory hurdles cleared, it’s time to bring the vision to life. Production processes are meticulously planned and optimized for larger quantities. This involves selecting the most efficient manufacturing techniques and establishing robust quality control measures to ensure the best customer experience.
For example, the company might settle for the printed electronics manufacturing technique, which involves printing electrical circuits directly onto flexible substrates. It’s ideal for creating lightweight and comfortable wearable devices like the Vitality Pro.
![]()
Key Considerations When Developing a Medical Device
Let’s explore some important considerations to ensure your prototyping experience goes smoothly.
- Market analysis: Conducting thorough market research helps determine the viability of your concept. This analysis identifies potential competitors and assesses the overall market size to ensure your device fills a real gap in the healthcare landscape.
- Cost and budget: Medical device prototyping can involve varying costs depending on the complexity of your design and the chosen methods. Be sure to factor in all potential costs upfront, including materials, labor, and testing. Many prototyping companies offer flexible solutions to fit your budget without compromising quality.
- Intellectual property (IP) protection: Consider safeguarding your innovative idea. Securing patents or other forms of intellectual property protection can be crucial to prevent unauthorized use of your design.
- Selecting the right prototyping partner: Choosing the right partner for your medical device prototyping journey is crucial. Look for a company with experience developing medical devices and a strong understanding of the regulatory landscape.
Examples of Successful Medical Device Prototypes
Here at StudioRed, we’ve had the privilege of collaborating with clients on a variety of medical devices. Our case studies showcase how real prototypes paved the way for successful medical products.
Labcyte

StudioRed partnered with Labcyte to develop the Labcyte Access Dual Robot System (DRS). This automated high-throughput platform integrates acoustic liquid dispensing technology that makes managing and storing lab samples much easier for laboratories.
Here’s how StudioRed helped Labcyte:
- Ergonomic design: We used anthropometric data to ensure the Access DRS met ergonomic standards and user comfort during operation and maintenance, including considerations for body positioning, shelf access, and the need for step stools.
- User interface considerations: We incorporated easy access for programming and servicing devices and positioned alert lights for optimal visibility from all angles.
- Modular design: We designed a modular system with user-friendly features and minimal parts.
- Custom component sourcing: Our engineering team sourced and specified custom environmental control ports for the robot arms, finding a creative solution for technical challenges.
Medtronic
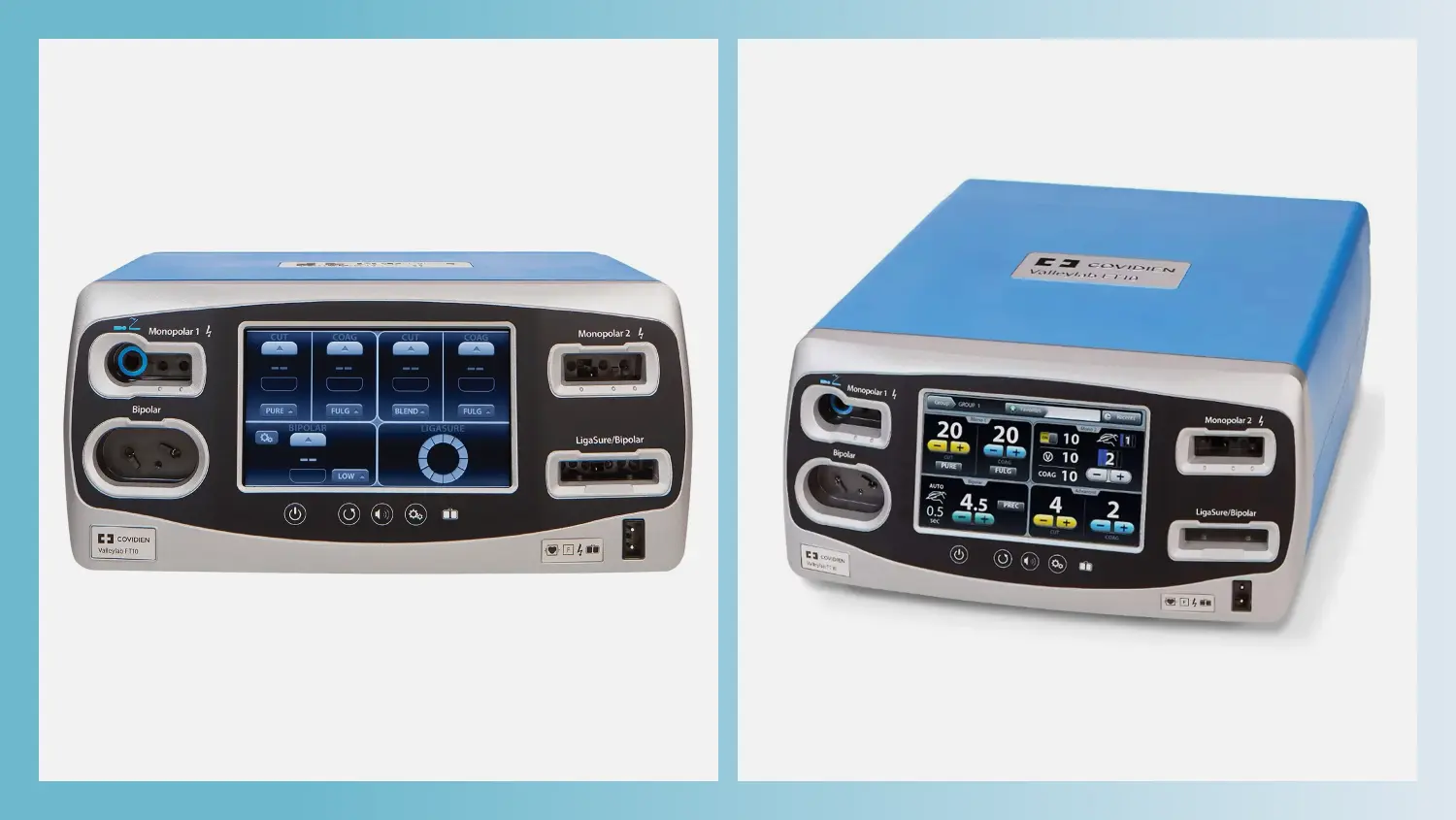
The Valleylab™ FT10 is an advanced surgical tool that uses precise electric cuts to minimize complications during surgery. It’s like a super-smart scalpel that ensures a clean and consistent cut. Our team was involved in designing the physical form and user interface of the Valleylab™ FT10.
Ready to turn your innovative medical device concept into reality? Our team of experienced industrial designers can bring your prototype to life. Contact us today!